
Supercapacitors
November 16, 2020In recent years, there have been significant advancements in the field of supercapacitor technology with regards to research, design and implementation that have influenced industries around the world. However, South Africa industry is due to the absence, this paper aims to clarify the differences between batteries (particularly the lithium-ion battery) and supercapacitors, and to provide guidance for choosing the proper device according to the application. Batteries are the solution for high energy density applications whereas supercapacitors are used in high power density applications where long cycle life and fast charge-discharge rates are needed. Currently, there exists a competition between these two technologies in the market. However, the two technologies can be utilized to complement each other to achieve the best results.
.
The ever-expanding population and the rise in standards of living have resulted in a sharp increase in consumption and demand for energy. This has left the energy sector facing difficult challenges that require exploration and implementation of novel technologies to solve. As a result, energy research has become an important area of focus in scientific communities around the world which has led to advancements in both energy production and storage technologies.
With regards to energy storage, there are two dominant technologies in use today. Batteries (such as lithium-ion or nickel–metal hydride) that have high energy density and supercapacitors (SCs) (electrochemical capacitors) that have high power density. However, due to the fact that batteries have existed for decades and are utilized for a wide range of applications, Most people and industries are better familiar with this technology compared to supercapacitors.
There are three technical characteristics that determine which specific energy storage technology should be selected for a particular application: energy density and power density which eventually determines the physical size of the storage system; charge and discharge time which must be harmonious with the proposed use, and cycle life which usually determines the operational life of an energy storage system. In some applications, other characteristics like maintenance requirements, operating temperature range, reliability, safety and cost have an importance equal to the technical characteristics. Environmental factors are frequently being considered, particularly in applications involving renewable energy like wind and solar, where the use of toxic or “non-green” materials in the storage system is highly inconvenient [1]. The aim of this article is to express in a clear fashion the differences between battery and SC technologies and how these two technologies can be used to complement each other in order to design better energy storage systems.
Supercapacitor Technology vs. Battery Technology
The fundamental differences between supercapacitors and batteries are in their constituent materials and the mechanisms involved in their charge and discharge operations. Batteries store energy chemically with much higher energy density than SCs. On the other hand, SCs store charges physically in the electric double layer at a surface-electrolyte interface with much higher power density and no significant change in the structure of the material with charge state. Therefore, an efficient, high performance, low cost and environmentally safe all-in-one energy storage system that combines the properties of the batteries and SCs is required. Furthermore, hybrid systems of the two devices might play complementary functions when they are combined with each other [2] and can give us a storage system with high energy and high power densities.
SCs can be classified into two categories based on their charge-storage mechanism and materials used for their fabrication, namely; electrical double-layer capacitors (EDLCs) and Pseudocapacitors (PCs) [5]. The EDLCs mainly focus on the use of different allotropes of carbon materials as electrode [4]–[6], while PCs using designate electrode materials such as metal oxides (RuO2, MnO2), and conducting polymers that have the electrochemical signature of a capacitive electrode [7]. SCs show linear shape during charge-discharge and they can reach 0 voltage without losing any capacity. However, by adding faradic material to the system, the energy density of SCs improve. Therefore, the moment that the charge-discharge shape of the system deviates from a straight line, we should call it hybrid electrochemical capacitor (HEC). The naming of the system is important because, SCs and HECs have both similar and different properties, therefore they must be designated properly.. Compared to SCs, HECs cycle life is shorter and the system doesn’t like to reach zero voltage (Although they can reach zero voltage, but it has a negative impact on their cycle life) (Fig. 2).
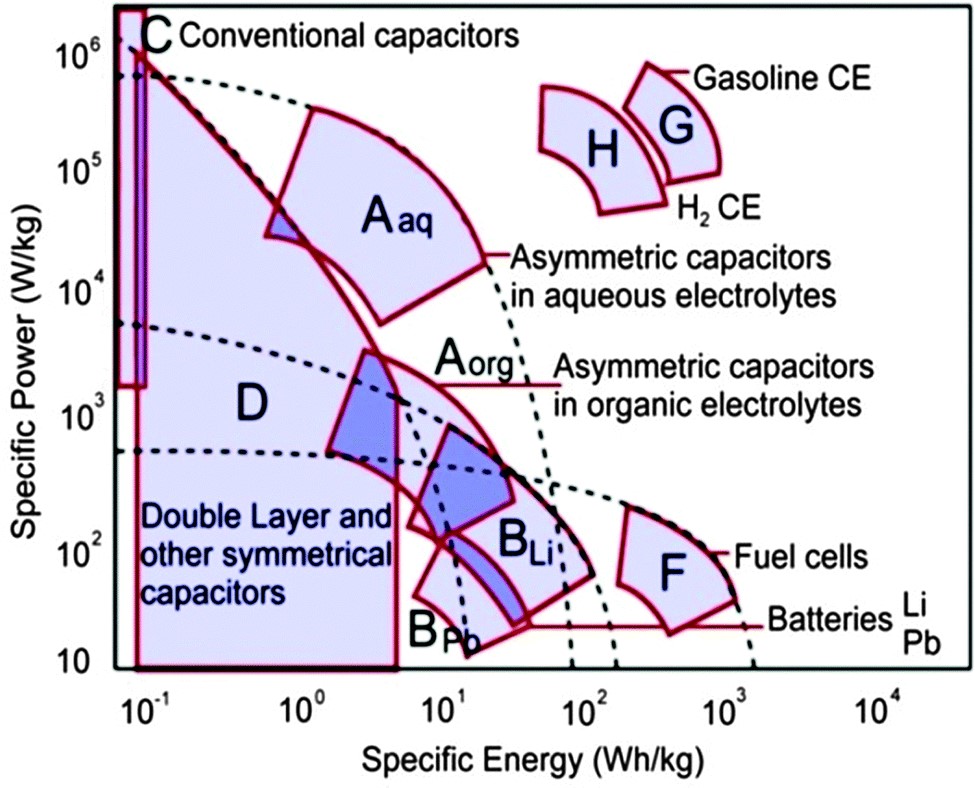
Fig. 1. Sketch of Ragone plot with specific energy and power for different energy storage devices [3]
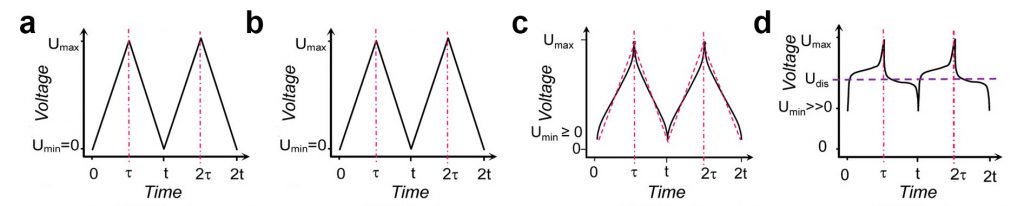
Fig. 2. Charge-discharge shape of (a) EDLC, (b) PC, (c) HEC and (d) Battery
Some important characteristics that must be thought through when comparing SCs and batteries are the energy density, power density, cycle life, the operative temperature limits, self-discharging, equivalent series resistance (ESR) and charge-discharge durations. It is accepted that the SCs are able to deliver higher specific powers when batteries have an advantage in higher specific energy. The cycle life of SCs can reach upto several million cycles, while batteries normally cannot reach to 2000 cycles without starting to degrade. Batteries have high ESR at cold temperatures with a very low self-discharging rate. However, SCs have Lower ESR even at cold temperatures with the high self-discharging rate. The operative temperature for SCs is -40 °C to +85 °C while the range for Lithium-ion batteries (and NiCd, NiMH) is 0 °C to ~+50 °C and for the Lead-acid battery is -20 °C to +50 °C. There are critical differences between SCs and batteries with regards to thermal management. In most applications, such as heavy hybrid vehicles, the charge-discharge cycles are quick and consist of very high power levels. Energy lost to heat during each cycle for SCs is relatively small and readily removed. However, the energy lost to heat in batteries is a much larger amount, making heat removal more crucial and costly [8].It is clear that SCs have some advantages over batteries such as virtually unlimited cycle life, high specific power; low ESR, with excellent low-temperature charge and discharge performance and safety. However, they have some limitations such as low specific energy (holds a fraction of the energy compared to a regular battery), linear discharge voltage prevents using the full energy spectrum, high self-discharge, low cell voltage (requires series connections with voltage balancing) and high cost per watt at the moment.
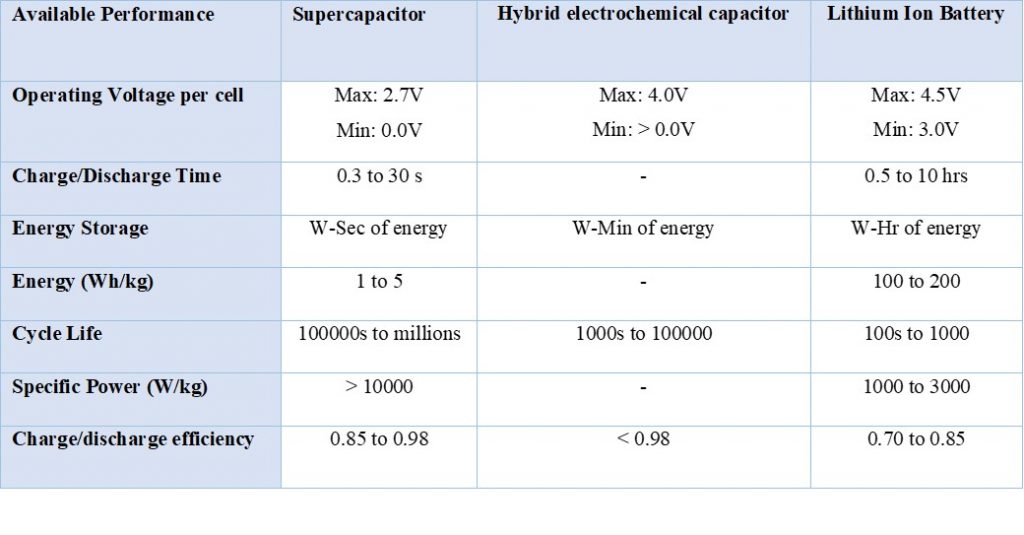
Table 1. Comparison of lithium ion batteries, Supercapacitors and hybrid electrochemical capacitor
Electrochemical capacitor application examples
SC applications can be ordered into four categories, namely; power, power support, memory back-up and energy storage. In the power section, SCs are used in wind turbines and solar tracking systems for the moving part of the structure. Also they are used is UPS’, golf carts and robotic arms and joints. Some of the applications in power support section include motor starters, hybrid cars, smart meters, controller and copy machines and digital cameras. As memory back-up, they are used in mobile phones, digital cameras, wireless devises and audio player systems. In the energy storage section, SCs can be found in road signs, solar energy systems, flashlights and some small electric devices such as solar watches and remote controls.
SCs can be a very good replacement for batteries for applications where a system requires backup power for a relatively short time (minutes) such as during the gap between switching to a different power supply (in the case of load shedding and starting a diesel generator). In addition, they are very good for applications in hot or cold environments where the batteries do not perform optimally. Mining industry can benefit from SCs in different applications. The Rockster R1100DE rock crusher shown in Fig. 3 (a) has a hybrid power system consisting of a diesel generator and SCs. Fuel savings of 30% along with 43% higher rock crushing throughput have been reported in comparison to conventional models, which require a significantly larger-sized engine to deliver the power peaks [1], [9]. Komatsu Hybrid machines shown in Fig. 3 (b) reduced fuel consumption and emissions of their products by up to 40% [10]. Fig. 3 (c) is a photograph of a Caterpillar 6120 H FS 1400-ton mining shovel. This system is powered by a hybrid diesel-electric system that has a 48 MJ energy storage system comprised of SCs. Regenerative energy storage occurs during swing deceleration and boom-down movements, reportedly leading to fuel savings of 25% over the non-hybrid version [1], [11]. In hybrid rubber tired gantry crane (RTGC) shown in Fig. 3 (d), a 7 MJ SC is used in the system resulting in up to 38% increase in fuel efficiency [12].

Fig. 3. Picture of the hybrid system with SCs (a) Rockster R1100DE, (b) Komatsu Hybrid machine, (c) Caterpillar 6120 H FS and (d) RTGC
Based on this brief report, we can conclude that batteries and supercapacitors have different properties that make each one suitable for a certain applications. Batteries have high charge density and therefore are able to provide power to systems for longer periods of time. However, they suffer from short cycle life and pose safety and environmental hazards.
Supercapacitors have high power density that makes them suitable for applications where high power is required for short durations of time. Also, due to their fast charge and discharge rates and their low internal resistance, they are the ideal devices for storing high current transient electrical surges to boost energy efficiency of a system. Finally, thanks to their environmentally friendly nature and long cycle life, use of supercapacitors can reduce costs associated with maintenance, disposal and global environmental taxes as well as reduction in environmental impacts of high powered systems
References
[1] J. R. Miller, “Engineering electrochemical capacitor applications,” J. Power Sources, vol. 326, pp. 726–735, 2016.
[2] F. Béguin, V. Presser, A. Balducci, and E. Frackowiak, “Carbons and electrolytes for advanced supercapacitors.,” Adv. Mater., vol. 26, no. 14, pp. 2219–2251, Apr. 2014.
[3] D. P. Dubal, O. Ayyad, V. Ruiz, and P. Gómez-Romero, “Hybrid energy storage: the merging of battery and supercapacitor chemistries.,” Chem. Soc. Rev., vol. 44, no. 7, pp. 1777–90, Apr. 2015.
[4] H. Wang et al., “Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy.,” ACS Nano, vol. 7, no. 6, pp. 5131–41, Jun. 2013.
[5] B. Abdulhakeem et al., “Morphological characterization and impedance spectroscopy study of porous 3D carbons based on graphene foam-PVA/phenol-formaldehyde resin composite as an electrode material for supercapacitors,” RSC Adv., vol. 4, no. 73, pp. 39066–39072, Aug. 2014.
[6] K. H. An, “Supercapacitors using singlewalled carbon nanotube electrodes,” in AIP Conference Proceedings, 2001, vol. 590, pp. 241–244.
[7] T. Brousse, D. Belanger, and J. W. Long, “To Be or Not To Be Pseudocapacitive?,” J. Electrochem. Soc., vol. 162, no. 5, pp. A5185–A5189, Mar. 2015.
[8] J. R. Miller and A. F. Burke, “Electrochemical capacitors: Challenges and opportunities for real-world applications,” Electrochem. Soc., vol. 17, pp. 53–57, 2008.
[9] “R1100DE track-mounted hybrid impact crusher.” https://www.rockster.at/content/en/produkte/r1100de_raupenmobiler_hybrid_prallbrecher.html. [01-Oct-2018].
[10] “Komatsu Hybrid Technology.” https://www.komatsu.eu/en/Komatsu-Hybrid-Technology. [01-Oct-2018].
[11] “Caterpillar announces development of largest hydraulic shovel.” https://www.cat.com/en_ZA/news/machine-press-releases/caterpillar-announcesdevelopmentoflargesthydraulicshovel.html. [03-Oct-2018].
[12] T. Furukawa, “DLCAP energy storage system multiple application,” in Adv. Capacitor World Summit, 2006.


